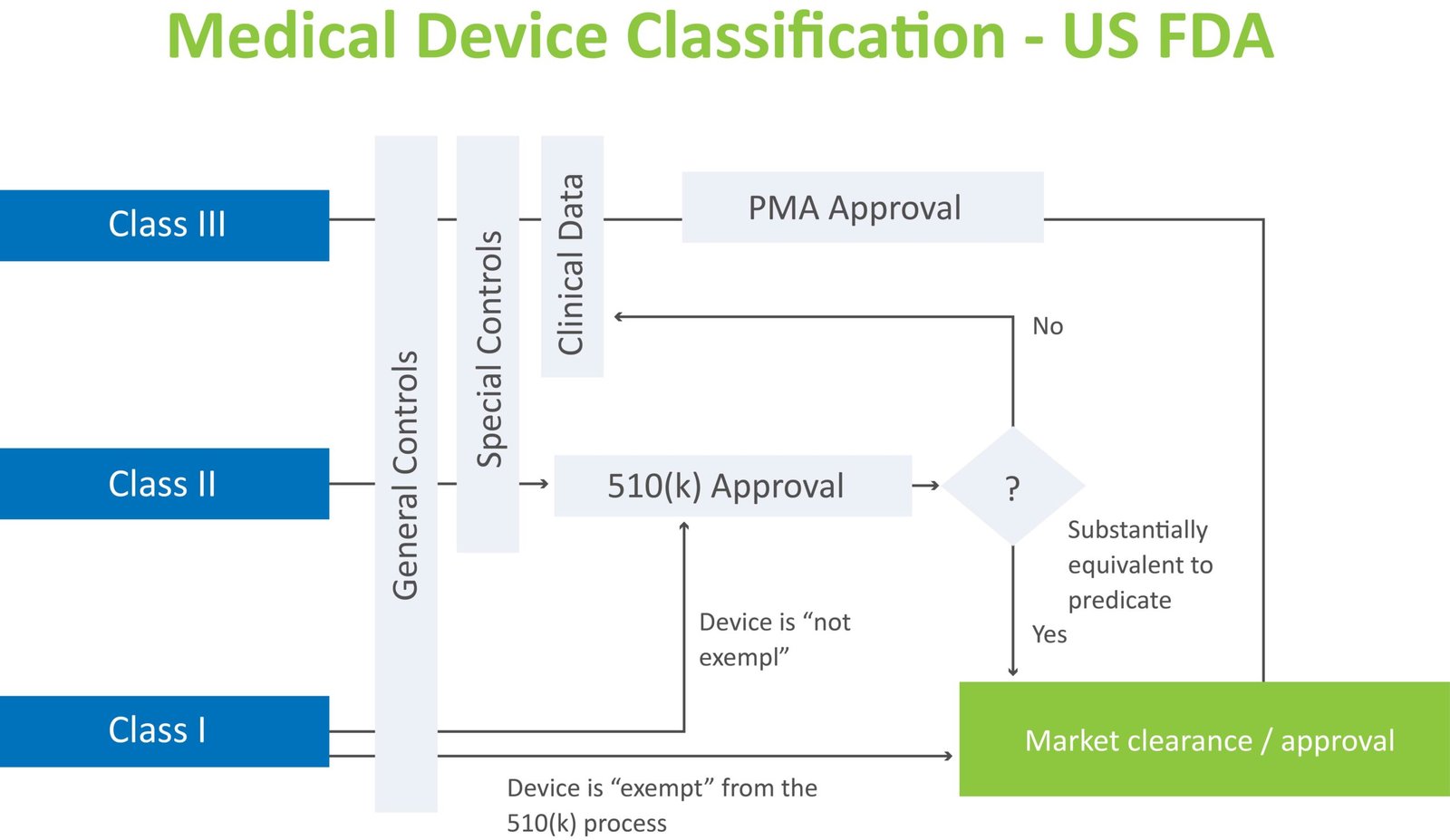
About 510(k)
A 510(k) is a premarketing submission made to FDA to demonstrate that the device to be marketed is as safe and effective, that is, substantially equivalent (SE), to a legally marketed device that is not subject to premarket approval (PMA). 510(k) (premarket notification) to FDA is required at least 90 days before marketing unless the device is exempt from 510(k) requirements.
510k Phases
- 1
-
2
Check your device is required 510k submission or 510k exempt
-
3
Get the 510k Total Product Life Cycle Report within 48 hours
(Review FDA decision letter and substantial device)
-
4
Prepare 510k submission
-
5
Submit 510k to FDA
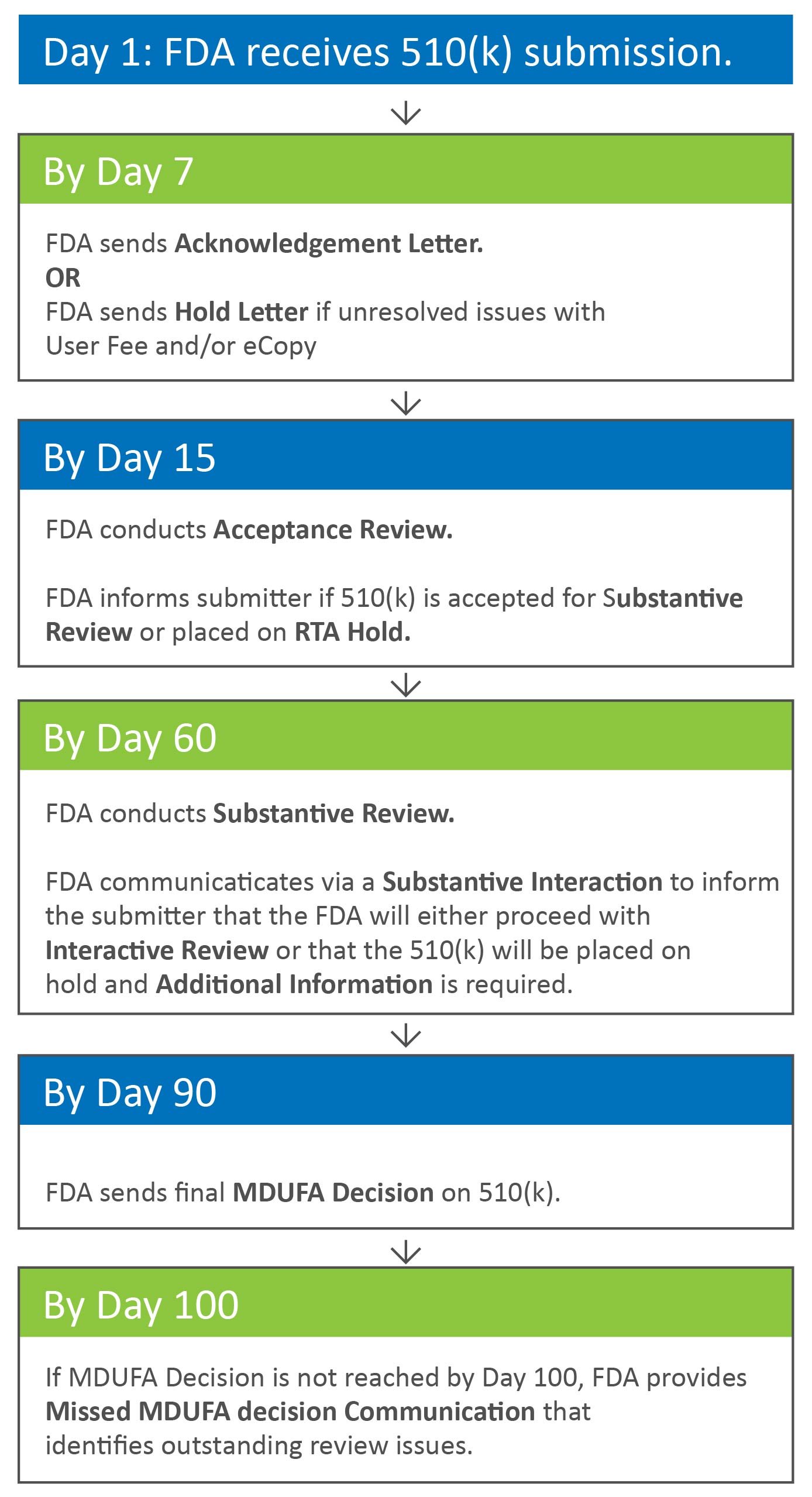
510k Submission Process FDA
510(k) Forms
List of forms associated with Premarket Notification (510[k]) submissions:
- Acceptance Checklists for Traditional, Abbreviated, and Special 510(k)s
- Premarket Notification Class III Certification and Summary
- Premarket Notification Truthful And Accurate Statement
- Premarket Notification 510(k) Statement
- Exempt Device Review Form (PDF – 16KB)
- 510(k) Cover Sheet Memorandum (PDF – 41KB)
- 510(k) “Substantial Equivalence” Decision Making Process (PDF – 844KB)
- Indications for Use (PDF – 1.7MB)
- Required Elements for a Declaration of Conformity to a Recognized Standard
Checklists for 510(k)
Refuse to Accept Policy for 510(k)s describes the criteria
FDA intends to use in assessing whether a 510(k) submission meets a minimum threshold of acceptability and should be accepted for substantive review.
The guidance includes acceptance checklists for each type of 510(k) submission:
- Traditional 510(k) Checklist
- Abbreviated 510(k) Checklist
- Special 510(k) Checklist
Here is Checklists for 510(k)
